Why do some soils disperse?
Should I care?….. Well, maybe…if you don’t want tunnels under your house or in your backgarden.
Most soils are normally aggregated, structured materials – this is due to soil constituents like carbon, calcium and iron oxide – they all join clay, silt and sand particles together to form soil aggregates (or peds). These clusters of soil materials help form the soil structure and provide resistance to erosion, healthy rooting media and pore spaces in-between the aggregates. These pores spaces can both hold water and also allow some of it to drain through. Note the good soil structure on the right (below) and the degraded soil structure on the left (due to over-cultivation and reduced soil carbon in the soil).

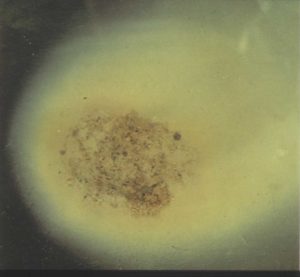
However, if the soil contains too much sodium, like in soaps and detergents, the clays become unstable and prone to separate apart i.e., disperse. By sodium, this means sodium ions; not salt or NaCl. In fact salt helps improve soil structure and aggregation as salts dry out the soil causing clays to cluster together. Sodium ions however are monovalent ions (+), the weakest charge possible, Ca ions are divalent (2+), Fe ions trivalent (3+); so sodium ions are the weakest of electrostatic magnets when it come to holding soil clays together. Note clays are mostly negatively charged – and opposites attract. But worse still, the sodium ions have very large water halos (hydration shells) around them, making the ion even weaker charged at its edge. This leads to clay particles (- charges) being only very weakly joined by sodium ions (+) as compared to calcium (2+), magnesium (2+) and aluminium (3+). See below a sodic clay aggregate dispersing – this happens when the soil is placed in distilled (pure) water e.g., rainwater.
Soils rich in sodium ions are called – Sodic soils or Sodosols. Sodic soil layers occur most typically in the clayey subsoils and lower layers of profiles in semi-arid areas of Tasmania and often are worse near the coast. These are areas where sodium ions are added to the soil via rainfall but because of high rates of evaporation in these dry areas the sodium ions are not washed all the way out of the soil profile and instead attach to the soil clays lower in the soil profile. SE Tasmania has many clayey soils with sodic sub-layers.
Do all sodic clays disperse? —- NO, not all! Why…….well other factors also affect dispersion like the soil organic matter, the clay types and the amount of iron oxides. Also the amount of sodium is important, therefore more sodium equals more dispersion.
Are some sodic soils more prone to dispersion than others?……….YES…..if the soil is a fine sandy clay or a silty clay then dispersion is more likely and more problematic. See some of the subsoil tunnels formed by underground dispersion in fine sandy clay soils formed above Triassic sandstones near Tea Tree; also the third photo shows subsoil dispersion at a building site on Triassic sandstone. The sandy clays allow for more ready dispersion and flow of water sideways through the soil and landscape, creating underground tunnels which eventual collapse inward and form gullies.



As well as causing tunneling and gully erosion and potentially undermining of infrastructure, sodic subsoil erosion also leads to sedimentation and decline in water quality – see the cloudy farm dam below (south of Kingston).

How do you manage sodic and dispersive soils? The answer is through ensuring topsoil covers the sodic or dispersive layers. If excavation is required then keep it to a minimum, do it in the drier part of the year and cover exposed dispersive soil with about 0.5 kg of gypsum (calcium sulfate) per square-metre to help prevent dispersion. The gypsum acts both as a salt and a source of more strongly clay bonding calcium.
